Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
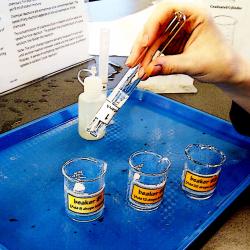
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution. Visitors observe the solutions to see which concentration of iodate reacts the most quickly. Learners come to understand that the speed of a chemical reaction is dependent on the concentration of the chemicals involved. This activity is currently used in the Chemical Reactions Unit in OMSI's Chemistry Lab. Cost estimates are per 100 learners. For safety reasons, this activity is best conducted as a demonstration for younger audiences.
- 30 to 45 minutes
- 5 to 10 minutes
- $1 - $5 per group of students
- Ages 14 - adult
- Activity, Demonstration, Experiment/Lab Activity
- English
Quick Guide
Materials List (per group of students)
- (3) 10-mL test tubes
- test tube rack
- (3) 50-mL beakers
- 10-mL graduated cylinder
- 30-mL dropper bottle
- 250-mL squeeze bottle
- 100g potato starch
- 100-mL 1M sulfuric acid (H2SO4)
- 25g sodium metabisulfite (Na2S2O5)
- 100g potassium iodate (KIO3)
Subjects
-
Physical Sciences
-
Chemistry
- Chemical Reactions
- Solutions
-
Chemistry
Audience
To use this activity, learners need to:
- see
- see color
- read
- touch
Learning styles supported:
- Involves hands-on or lab activities
Other
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, Oregon Museum of Science and Industry, 1997
Funding Source:
- National Science Foundation, 9355628
