Search Results
Showing results 1 to 20 of 22

Candy Chemosynthesis
Source Institutions
In this activity, groups of learners work together to create edible models of chemicals involved in autotrophic nutrition.
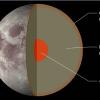
Recipe for a Moon
Source Institutions
In this activity, learners discover that the Moon, like Earth, is made up of layers of different materials. Learners work in teams to make models of the interiors of the Moon and Earth.

Bury Me Not!
Source Institutions
This activity (page 2 of the PDF under SciGirls Activity: Bogs) is a full inquiry investigation into decomposition.

Pie-Pan Convection
Source Institutions
It's difficult to see convection currents in any liquid that's undergoing a temperature change, but in this Exploratorium Science Snack, you can see the currents with the help of food coloring.

Chocolate (Sea Floor) Lava
Source Institutions
In this edible experiment, learners pour "Magic Shell" chocolate into a glass of cold water. They'll observe as pillow shaped structures form, which resemble lavas on the sea floor.

Hot Sauce Hot Spots
Source Institutions
In this activity, learners model hot spot island formation, orientation and progression with condiments.

Geyser
Source Institutions
This Exploratorium activity can be used in many contexts because geysers are great opportunities for learning about heat and temperature changes as well as geological/space science phenomena.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Freezing Lakes
Source Institutions
In some parts of the world, lakes freeze during winter. In this activity learners will explore water’s unique properties of freezing and melting, and how these relate to density and temperature.

How Boulders Are Born
Source Institutions
In this activity, learners review and discuss weathering, erosion and mass wasting, to gain a stronger understanding of how Hickory Run’s Boulder Field was formed after the Laurentide Continental Glac
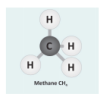
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

Diet Light
Source Institutions
In this quick activity, learners observe how the added sugar in a can of soda affects its density and thus, its ability to float in water.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Melts in Your Bag, Not in Your Hand
Source Institutions
In this activity, learners use chocolate to explore how the Sun transfers heat to the Earth through radiation.

Making Connections: What You Can Do To Help Stop Global Climate Change
Source Institutions
In this cooperative learning activity, learners visit ten stations and are challenged to think critically about various conservation questions and issues.

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

We all Scream for Ice Cream
Source Institutions
In this activity, learners observe how salinity affects the freezing point of water by making and enjoying ice cream.
Trash Talkin'
In this activity, learners collect, categorize, weigh and analyze classroom trash and discuss ways that engineers have helped to reduce solid waste.

I Can't Take the Pressure!
Learners develop an understanding of air pressure in two different activities.
