Search Results
Showing results 1 to 20 of 26
Big Bubbles
Source Institutions
How do you measure a bubble when it's floating? You can't really, but in this activity, learners can measure the diameter of the ring of suds a bubble leaves on a flat surface.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.

Does Air Weigh Anything?
Source Institutions
The demonstration/experiment provides quick proof that air has mass.

Change in Temperature: Exothermic Reaction
Source Institutions
Learners add calcium chloride to a baking soda solution and observe an increase in temperature along with the production of a gas and a white precipitate. These are all signs of a chemical reaction.

Measure the Pressure II: The "Dry" Barometer
Source Institutions
In this activity, learners use simple items to construct a device for indicating air pressure changes.

Inflate-a-mole
Source Institutions
In this activity, learners conduct an experiment to find the volume of one mole of gas. Learners capture sublimated gas from dry ice in a ziploc bag and use water displacement to measure its volume.
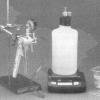
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

What Causes Pressure?
Source Institutions
In this kinesthetic activity that demonstrates pressure, learners act as air molecules in a "container" as defined by a rope.
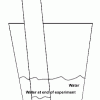
Percentage of Oxygen in the Air
Source Institutions
In this activity, learners calculate the percentage of oxygen in the atmosphere by using steel wool's ability to rust.

Mold Mole Molds
Source Institutions
In this activity, learners make different shapes that hold exactly one mole of gas (air).

Earth Atmosphere Composition
Source Institutions
In this activity, learners use rice grains to model the composition of the atmosphere of the Earth today and in 1880. Learners assemble the model while measuring percentages.
Twirling in the Breeze
Source Institutions
In this engineering activity, learners build a device (an anemometer) to measure how fast the wind is blowing.

Atmosphere Composition Model
Source Institutions
In this activity, learners create a model using metric measuring tapes and atmosphere composition data.

Conservation of Mass
Source Institutions
This activity was designed for blind learners, but all types of learners can participate to learn about conservation of gas. This is one of the classic experiments using baking soda and vinegar.

Dunking the Planets
Source Institutions
In this demonstration, learners compare the relative sizes and masses of scale models of the planets as represented by fruits and other foods.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

Weather Vane and Anemometer
Source Institutions
In this meteorology activity, learners construct simple devices to measure the direction and speed of wind.

Production of a Gas: Controlling a Chemical Reaction
Source Institutions
Learners mix vinegar and baking soda to produce a gas. With the addition of a bit of liquid soap, the gas becomes trapped in measurable bubbles.

