Search Results
Showing results 1 to 20 of 40

Walk On Water Bugs
Source Institutions
In this activity (on pages 29-35), learners examine water pollution and filtration.

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.
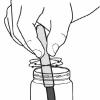
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.
Can Nutrients in Water Cause Harm?
Source Institutions
In this water pollution activity, learners create pond water cultures and investigate the effects of adding chemicals or natural nutrients.

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Release the Grease!
Source Institutions
In this simple activity (on page 7 of the PDF), learners use water and liquid dish detergent to see which one removes lipstick better from an index card.

Exploring Size: Ball Sorter
Source Institutions
In this activity, learners use sieves with different-sized holes to sort balls by size.
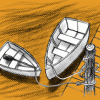
Watercraft
Source Institutions
In this design challenge activity, learners build a boat that can hold 25 pennies (or 15 one inch metal washers) for at least ten seconds before sinking.

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Fragile Waters
Source Institutions
In this activity (on pages 18-29) learners explore the impact of the March 24, 1989 oil spill in Alaska caused by the Exxon Valdez tanker.
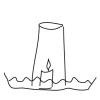
Thirsty Candle
Source Institutions
In this activity, learners will explore the dynamics of air pressure by using a candle, a cup, and a dish of water.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Exploring Materials: Hydrogel
Source Institutions
In this activity, learners discover how a super-absorbing material can be used to move a straw.

Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.
