Search Results
Showing results 1 to 20 of 61

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Walk On Water Bugs
Source Institutions
In this activity (on pages 29-35), learners examine water pollution and filtration.

Breaking the Tension: Surface Tension 1
Source Institutions
Learners explore how the attractive forces between water molecules create surface tension and allow certain objects to float on the surface of water.

Moving On Up: Capillary Action 1
Source Institutions
Over the course of several days, learners explore the property of water that helps plants move water from roots to leaves or gives paper towels the capacity to soak up water.
How Does Water Climb a Tree?
Source Institutions
In this activity, learners conduct an experiment to explore how water flows up from a tree's roots to its leafy crown.

Amazon Water Cycle Roleplay
Source Institutions
In this creative roleplay activity, learners will explore the various processes of the water cycle using movement, sound, and props to aid in comprehension.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.
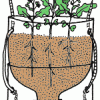
TerrAqua Investigation Column: What is the Land-Water Connection?
Source Institutions
In this investigation, learners plant seeds in a 2-liter bottle filled with soil that is connected to a water source below. Over the next few weeks, learners observe how the plants grow.
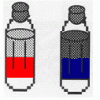
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Color-Changing Carnations
Source Institutions
Learners place cut flowers in colored water and observe how the flowers change. The flowers absorb the water through the stem and leaves.

Water Cycle in a Bag
Source Institutions
In this activity, learners create a biosphere in a baggie.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Turbidity
Source Institutions
This is an activity about turbidity, or the amount of sediment suspended in water.

Oh Boy Buoyancy
Source Institutions
In this physics activity, learners will explore the concept of buoyancy, especially as it relates to density.

Water "Digs" It!
Source Institutions
In this activity, learners investigate soil erosion. Learners set up a simulation to observe how water can change the land and move nutrients from one place to another.

Water Sphere Lens
Source Institutions
In this activity about light and refraction, learners make a lens and magnifying glass by filling a bowl with water.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Portable Potable Pressure
Source Institutions
In this activity, learners use plastic water bottles, wood, and water to build an inexpensive and portable tool to demonstrate one atmosphere of pressure at sea level.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).
