Search Results
Showing results 1 to 20 of 55

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Water, Water Everywhere
Source Institutions
In this activity, learners estimate how much water they think can be found in various locations on the Earth in all its states (solid, liquid, and gas) to discover the different water ratios in the Ea

Storm Water Runoff Pollution
Source Institutions
This activity (located on page 8 of the PDF) introduces learners to the concept of Non-point Source Pollution--what happens when rain washes garbage and other pollutants into rivers and lakes.
Pepper Scatter
Source Institutions
In this activity, learners explore the forces at work in water. Learners experiment to find out what happens to pepper in water when they touch it with bar soap and liquid detergent.

Make a Salt Volcano (Lava Lite)
Source Institutions
This activity about density provides instructions for making a miniature "lava lite" with just salt, oil, water, and food coloring.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.
Dollar Bill Grab
Source Institutions
In this demonstration, learners observe as two cola bottles and a dollar bill are arranged in a specific order: one bottle, upside down and filled with water, is placed on top of another bottle, with

The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.
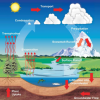
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Diet Light
Source Institutions
In this quick activity, learners observe how the added sugar in a can of soda affects its density and thus, its ability to float in water.

Do Sweat It!
Source Institutions
In this activity, learners explore why humans sweat. Learners compare the effects of heat on a balloon filled with air and a balloon filled water.

Exploring Size: Ball Sorter
Source Institutions
In this activity, learners use sieves with different-sized holes to sort balls by size.

Defining Density
Source Institutions
In this introductory demonstration and activity, learners are introduced to the concept of density as they explore a rock and a wooden block in water.

Why Doesn’t the Ocean Freeze?
Source Institutions
In this activity, learners explore how salt water freezes in comparison to fresh water.

Atmospheric Collisions
Source Institutions
In this activity/demonstration, learners observe what happens when two ping pong balls are suspended in the air by a hair dryer. Use this activity to demonstrate how rain drops grow by coalescence.

Fog Chamber
Source Institutions
In this weather-related activity, learners make a portable cloud in a bottle.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Water on the Move: Wind and Waves
Source Institutions
In this simple activity, learners explore ocean waves. To find out if water moves forward toward the shore, learners create waves in a simulated ocean (small aquarium tank of water).
Make Your Own Deep-Sea Vent
Source Institutions
In this activity, learners make a model of the hot water of a deep sea vent in the cold water of the ocean to learn about one of the ocean's most amazing and bizarre underwater habitats.
Let's Go Ice Fishing
Source Institutions
In this activity, learners are challenged to lift a floating ice cube out of a glass of water using just one end of a piece of string.
