Search Results
Showing results 1 to 20 of 80

The Amazing Water Trick
Source Institutions
Using two baby food jars, food coloring, and an index card, you'll 'marry' the jars to see how hot water and cold water mix.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.

Heat Capacity: Can't Take the Heat?
Source Institutions
Why is ocean water sometimes the warmest when the average daily air temperature starts to drop? In this activity, learners explore the differing heat capacities of water and air using real data.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Make a Salt Volcano (Lava Lite)
Source Institutions
This activity about density provides instructions for making a miniature "lava lite" with just salt, oil, water, and food coloring.

Make a Water Cycle Wristband
Source Institutions
In this activity, learners thread colored beads onto string. Each beach represent a process of the water cycle.

Solar Water Heater
Learners work in teams to design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy.

Water Cycle in a Bag
Source Institutions
In this activity, learners will explore the water cycle by creating a small atmosphere.

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.
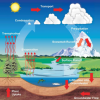
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Do Sweat It!
Source Institutions
In this activity, learners explore why humans sweat. Learners compare the effects of heat on a balloon filled with air and a balloon filled water.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Why Doesn’t the Ocean Freeze?
Source Institutions
In this activity, learners explore how salt water freezes in comparison to fresh water.

Atmospheric Collisions
Source Institutions
In this activity/demonstration, learners observe what happens when two ping pong balls are suspended in the air by a hair dryer. Use this activity to demonstrate how rain drops grow by coalescence.

Solar Convection
Source Institutions
In this activity, learners add food coloring to hot and cold water in order to see how fluids at different temperatures move around in convection currents.

Fog Chamber
Source Institutions
In this weather-related activity, learners make a portable cloud in a bottle.
Make Your Own Deep-Sea Vent
Source Institutions
In this activity, learners make a model of the hot water of a deep sea vent in the cold water of the ocean to learn about one of the ocean's most amazing and bizarre underwater habitats.
