Search Results
Showing results 1 to 20 of 29

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.
Drip Drop
Source Institutions
In this water activity, learners explore how water drops behave on different surfaces.
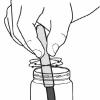
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

Sink or Float?
Source Institutions
In this water activity, learners test which objects float and which sink. Learners discover that objects behave differently in water.
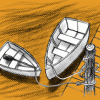
Watercraft
Source Institutions
In this design challenge activity, learners build a boat that can hold 25 pennies (or 15 one inch metal washers) for at least ten seconds before sinking.

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Boats Afloat
Source Institutions
In this water activity, learners build boats that float and sink. First, learners listen to the book, "Who Sank the Boat" and practice making predictions throughout the story.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Cauldron Bubbles
Source Institutions
In this activity, learners mix up a bubbly brew and examine density. Learners explore how they can make different materials fall and rise in water using oil, water, and salt.

Exploring Materials: Hydrogel
Source Institutions
In this activity, learners discover how a super-absorbing material can be used to move a straw.

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.

Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Color Splash
Source Institutions
In this activity, learners mix water, cooking oil, and liquid food coloring to create beautiful colored designs in a cup. Use this activity to explore liquid density and solubility.

Special Effects Using Household Chemicals
Source Institutions
In this activity on page 4 of the PDF (Behind the Scenes with Chemistry), learners make some special effects, including snow and breaking glass, with supplies found in the home.

Flinker
Source Institutions
In this activity, learners make a foam packing peanut "flink"--neither float away nor sink--in water. Learners experiment with materials to make a Flinker that "flinks" for 10 seconds.
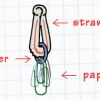
Deep Sea Diver
Source Institutions
In this ocean engineering activity, learners explore buoyancy and water displacement. Then, learners design models of deep sea divers that are neutrally buoyant.
