Search Results
Showing results 1 to 20 of 114

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Water, Water Everywhere
Source Institutions
In this activity, learners estimate how much water they think can be found in various locations on the Earth in all its states (solid, liquid, and gas) to discover the different water ratios in the Ea

Comparing the Density of an Object to the Density of Water
Source Institutions
Learners compare the weight of equal volumes of wax, water, and clay. Learners discover that since the wax weighs less than an equal volume of water, it is less dense than water and will float.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,

Disappearing Crystals
Source Institutions
Learners experiment with water gel crystals, or sodium polyacrylate crystals, which absorb hundreds of times their weight in water. When in pure water, the water gel crystals cannot be seen.

Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.
Big and Little Cups
Source Institutions
In this indoor or outdoor water activity, learners pour water from small cups to large cups and containers. In doing so, they discover water takes the shape of its container.

Measure the Speed of a Water Leak
Source Institutions
In this activity (page 2 of PDF under GPS: Glaciers Activity), learners will measure the rate at which water streams out of a leaky cup.

Solar Water Heater
Learners work in teams to design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy.
Making Rivers
Source Institutions
In this outdoor water activity, learners explore how to change the direction of water flow. Learners make puddles in dirt or use existing puddles and sticks to make water flow.

PVC Water Squirter
Source Institutions
In this activity, learners build a water squirter using a PVC pipe, dowel, and foam. This activity is great for the summer time and introduces learners to forces and water pressure.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!
Under Pressure
Source Institutions
In this experiment, learners examine how pressure affects water flow. In small groups, learners work with water and a soda bottle, and then relate their findings to pressure in the deep ocean.
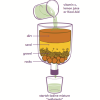
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

Science at the Waterpark!
Source Institutions
This activity (on page 2 of the PDF under SciGirls Activity: Water Slides) is a full inquiry investigation into speed and motion and takes place at a water park.

Liquid Lens
Source Institutions
In this activity, learners discover that they can create a lens from a water drop. Learners test their lens by looking at words or pictures.

Forces at the Nanoscale: Nano Properties of Everyday Plants
Source Institutions
This is an activity (located on page 3 of PDF under Nasturtium Leaves Activity) about surface tension.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).
