Search Results
Showing results 1 to 20 of 58

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,

Disappearing Crystals
Source Institutions
Learners experiment with water gel crystals, or sodium polyacrylate crystals, which absorb hundreds of times their weight in water. When in pure water, the water gel crystals cannot be seen.

Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.
What's So Special about Water: Absorption
Source Institutions
In this activity about water's cohesive and adhesive properties and why water molecules are attracted to each other, learners test if objects repel or absorb water.

What's So Special about Water: Surface Tension
Source Institutions
In this three-part activity, learners play a game and conduct two simple experiments to explore water and surface tension. Learners will have fun discovering how water "sticks" together.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!
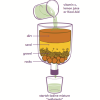
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Forces at the Nanoscale: Nano Properties of Everyday Plants
Source Institutions
This is an activity (located on page 3 of PDF under Nasturtium Leaves Activity) about surface tension.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

What's Hiding in the Air?: Acid Rain Activity
As a model of acid rain, learners water plants with three different solutions: water only, vinegar only, vinegar-water mixture.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.

Drops on a Penny
Source Institutions
In this activity, challenge learners to predict and investigate how many water drops they can fit on one penny.
Investigating Density Currents
Source Institutions
In this lab activity, learners explore how to initiate a density current. Learners measure six flasks with different concentrations of salt and water (colored blue).

Turbidity
Source Institutions
This is an activity about turbidity, or the amount of sediment suspended in water.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Nutrients in an Estuary
Source Institutions
In this activity, learners model estuaries, artificially enriching both fresh and salt water samples with different amounts of nutrients and observing the growth of algae over several weeks.
