Search Results
Showing results 1 to 20 of 52

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.

Levity Through Tension: Fun with Water's Surface Tension
Source Institutions
This experiment describes how to create a "dribble bottle" which only leaks water when the cap is unscrewed. The full water bottle has a small hole made with a push pin.

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,

Stuck on You: Adhesion
Source Institutions
Learners explore water adhesion and learn about why water molecules are more strongly attracted to some substances than others.
Cohesion Coin
Source Institutions
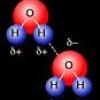
In this activity about the property of water (page 6 of the PDF), learners use a coin to demonstrate cohesion between water molecules, exploring the molecular forces that allow water molecules to "
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

Stick to It: Adhesion II
Source Institutions
Water sticks to all kinds of things in nature — flowers, leaves, spider webs - and doesn't stick to others, such as a duck's back.
Floating Paperclip and Other Surface Tension Experiments
Source Institutions
In this activity, learners experiment with surface tension using everyday household items such as strawberry baskets, paperclips, liquid dish soap, and pepper.

Crystal Creations: Grow Spikes of Crystals in the Sun
Source Institutions
This activity shows you how to make amazing crystal spikes using Epsom salt and the sun.

Forces at the Nanoscale: Nano Properties of Everyday Plants
Source Institutions
This is an activity (located on page 3 of PDF under Nasturtium Leaves Activity) about surface tension.

Surface Tension Icebreaker
Source Institutions
This is a quick activity (located on page 2 of the PDF under Nasturtium Leaves Activity) about surface tension.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.
Marshmallow Models
Source Institutions
No glue is needed for learners of any age to become marshmallow architects or engineers.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Exploring Materials: Hydrogel
Source Institutions
In this activity, learners discover how a super-absorbing material can be used to move a straw.
