Search Results
Showing results 1 to 20 of 88

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Water, Water Everywhere
Source Institutions
In this activity, learners estimate how much water they think can be found in various locations on the Earth in all its states (solid, liquid, and gas) to discover the different water ratios in the Ea

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).

Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.

What-a-cycle
Source Institutions
In this activity, learners act as water molecules and travel through parts of the water cycle to discover that it is more complex than just water moving from the ground to the atmosphere.
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

Frosty Glasses
Source Institutions
In this activity, learners explore why frost forms. They create their own frost using a solution of ice water and salt in a glass.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Make a Salt Volcano (Lava Lite)
Source Institutions
This activity about density provides instructions for making a miniature "lava lite" with just salt, oil, water, and food coloring.

Sinking Water
Source Institutions
In this experiment, learners float colored ice cubes in hot and cold water.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.
Instant Ice
Source Institutions
In this activity, learners observe a quick phase change as water rapidly goes from a liquid state to a solid state.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.

The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.
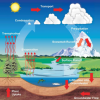
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Crystal Creations: Grow Spikes of Crystals in the Sun
Source Institutions
This activity shows you how to make amazing crystal spikes using Epsom salt and the sun.

Water Body Salinities II
Source Institutions
In this activity, learners discuss the different salinities of oceans, rivers and estuaries.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.
