Search Results
Showing results 1 to 19 of 19
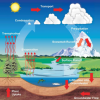
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!
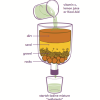
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.
The Dead Zone: A Marine Horror Story
Source Institutions
In this environmental science and data analysis activity, learners work in groups to track a Dead Zone (decreased dissolved oxygen content of a body of water) using water quality data from the Nutrien

Crystal Stencil Stars
Source Institutions
In this activity on page 6 of the PDF, learners dissolve Epsom salt in water and discover that the resulting solution can be used to create a work of art.

Hollandaise Sauce: Emulsion at Work
Source Institutions
In this activity, learners follow a recipe to make hollandaise sauce. Learners discover how cooks use egg yolks to blend oil and water together into a smooth mix.

Building Molecules
Source Institutions
This online interactive has three activities in the NanoLab (press the upper right button): Build, Zoom, and Transform.

Earth Atmosphere Composition
Source Institutions
In this activity, learners use rice grains to model the composition of the atmosphere of the Earth today and in 1880. Learners assemble the model while measuring percentages.

Mystery Matter
Source Institutions
This interactive demonstration reintroduces learners to three states of matter (solid, liquid, gas), and introduces them to a fourth state of matter, plasma.
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

It's a Gas, Man
Source Institutions
In this activity, learners discover if carbon dioxide has an effect on temperature.

Cook Up a Comet
Source Institutions
In this activity (on page 5 of PDF), learners use dry ice and household materials to make scientifically accurate models of comets.
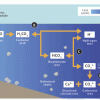
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.
