Search Results
Showing results 1 to 20 of 82

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Water Treatment
Source Institutions
Water treatment on a large scale enables the supply of clean drinking water to communities.
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Foam Tower
Source Institutions
In this activity (page 1 of the PDF under SciGirls Activity: Water Slides), learners will whip up some suds with a cup of water and a tablespoon of dish soap until the bubbles are stiff enough to star
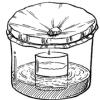
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.
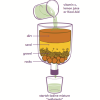
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Forces at the Nanoscale: Nano Properties of Everyday Plants
Source Institutions
This is an activity (located on page 3 of PDF under Nasturtium Leaves Activity) about surface tension.

Surface Tension Icebreaker
Source Institutions
This is a quick activity (located on page 2 of the PDF under Nasturtium Leaves Activity) about surface tension.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

What's Hiding in the Air?: Acid Rain Activity
As a model of acid rain, learners water plants with three different solutions: water only, vinegar only, vinegar-water mixture.

Moving Without Wheels
In a class demonstration, learners observe a simple water cycle model to better understand its role in pollutant transport.

Fragile Waters
Source Institutions
In this activity (on pages 18-29) learners explore the impact of the March 24, 1989 oil spill in Alaska caused by the Exxon Valdez tanker.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.
Marshmallow Models
Source Institutions
No glue is needed for learners of any age to become marshmallow architects or engineers.
