Search Results
Showing results 641 to 660 of 1185

Special Effects Using Household Chemicals
Source Institutions
In this activity on page 4 of the PDF (Behind the Scenes with Chemistry), learners make some special effects, including snow and breaking glass, with supplies found in the home.

Making Sodium Acetate: Hot Ice
Source Institutions
In this chemistry activity which should only be done under adult supervision (page 10 of the PDF), learners will create an exothermic process by making Sodium Acetate.

Super Soaking Materials
Source Institutions
In this activity, learners will test cups full of potting soil, sand, and sphagnum moss to see which earth material is able to soak up the most water.

Size it Up
Source Institutions
Learners investigate why the Sun and Moon appear the same size in the sky even though the Sun is over 400 times larger in diameter.
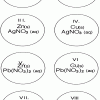
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

Drip, Drop, Drip, Drop
Source Institutions
In this math lesson, learners design an experiment to model a leaky faucet and determine the amount of water wasted due to the leak.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Challenge: Microgravity
Source Institutions
In this activity about the circulatory system and space travel (on page 38 of the PDF), learners use water balloons to simulate the effects of gravity and microgravity on fluid distribution in the bod

Below the Surface: Surface Tension II
Source Institutions
In this activity learners explore surface tension. Why are certain objects able to float on the surface of water and how do detergents break the surface tension of water?
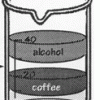
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Examining the Heart
Source Institutions
In this activity about the heart (on page 22 of the PDF), learners examine sheep or chicken hearts to learn about the heart's structure and the flow of blood through the heart.

Salt Water Revival
Source Institutions
In this outdoor activity, learners visit the intertidal zone of a rocky coastal site well populated with marine organisms.

Off Base
Source Institutions
In this activity, learners explore the factors that tend to resist changes in pH of the ocean and why the ocean is becoming more acidic.

I Can't Take the Pressure!
Learners develop an understanding of air pressure in two different activities.

Delicious Smelling Chemistry
Source Institutions
In this activity, learners use household materials to investigate and explore their ability to smell an odor.

What's Hiding in the Air?: Rubber Band Air Test
Learners build devices from rubber bands to test for invisible air pollutants.

Biomimicry: Synthetic Gecko Tape Through Nanomolding
Source Institutions
In this activity/demo, learners examine a synthetic gecko tape with micron sized hairs that mimics the behavior of the gecko foot.

Use Clues to Solve an Ice Mystery
Source Institutions
Learners explore the variables that affect the properties of ice and the places where different types of ice are found.

Bent Toward Science: Refraction
Source Institutions
This is an activity about the behavior of light. Using simple, everyday objects, learners will discover that light moves in straight lines until acted upon by another object.

Recycling Rules: Understanding Recycling and a MRF
Source Institutions
In this activity, learners simulate the separation techniques that materials recovery facilities (MRFs) use and then design their own series of recycling techniques.
