Search Results
Showing results 81 to 90 of 90

A Closer Look at Crystals
Source Institutions
In this activity, learners compare different types of salt crystals under a magnifying glass.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

Production of a Gas: Controlling a Chemical Reaction
Source Institutions
Learners mix vinegar and baking soda to produce a gas. With the addition of a bit of liquid soap, the gas becomes trapped in measurable bubbles.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.
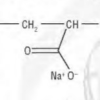
Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.
Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.

Change in Temperature: Exothermic Reaction
Source Institutions
Learners add calcium chloride to a baking soda solution and observe an increase in temperature along with the production of a gas and a white precipitate. These are all signs of a chemical reaction.
