Search Results
Showing results 1 to 8 of 8

Tiny Tubes
Source Institutions
In this activity, learners make "totally tubular" forms of carbon. Learners use chicken wire to build macro models of carbon nanotubes.

Water Quality and pH Levels in Aquatic Ecosystems
Source Institutions
In this fun and in depth hands-on experiment, learners test various liquid samples (distilled water, lemon juice, vinegar, and baking soda mixed with water) to determine their pH levels and identify e

Thar She Glows!
Source Institutions
Learners observe glow-in-the-dark objects in a homemade light-proof box. Objects can include glow sticks, glow-in-the-dark toys, and toys with fluorescent paint.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Fireworks!
Source Institutions
In this chemistry lab activity, learners model the colors of fireworks by burning metallic solutions in a flame and observing the different colors produced.
Light Quest
Source Institutions
Learners test their "light-smarts" by playing a game called "Light Quest!" The game board represents an atom and each player represents an electron that has been bumped into the atom's outer unstable
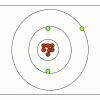
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Atomic Mobile
Source Institutions
Learners make a mobile model of a carbon atom using clay, wire, and pipe cleaners.
