Search Results
Showing results 1 to 20 of 23

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Red, White and Blue I Demonstration
Source Institutions
In this chemistry demonstration, learners observe a chemical reaction that produces a colorful effect.

Finding Red
Source Institutions
In this chemistry challenge, learners systematically investigate which combination of four solutions produces a deep red color.

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Magic Inks
Source Institutions
Learners write their initials by applying different clear "magic ink" solutions to separate pieces of paper and then "develop" the inks with other clear solutions.
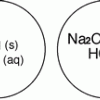
Gas Producing Micro-Reaction
Source Institutions
In this chemistry activity, learners use common chemicals and metals to examine reactions that produce gaseous substances.

Production of Oxygen
Source Institutions
In this chemistry activity, learners use yeast and hydrogen peroxide to generate a gas (oxygen) and test some of its properties.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Production of Hydrogen
Source Institutions
In this chemistry activity, learners use mossy zinc (or a galvanized nail) and hydrochloric acid to generate hydrogen gas and test some of its properties.
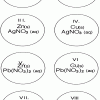
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.
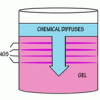
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.

Making Naked Eggs: Eggs Without Shells
Source Institutions
This is an activity about acid-base reactions using eggs and vinegar. Learners place eggs inside a container of vinegar and leave to soak overnight.

Production of Carbon Dioxide
Source Institutions
In this chemistry activity, learners use common chemicals to produce carbon dioxide and observe its properties. This resource includes brief questions for learners to answer after the experiment.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.

Pop Rockets
Source Institutions
Learners place water and part of an antacid tablet in a film canister. The reaction creates a gas reaction that launches the film canister like a rocket.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.
