Search Results
Showing results 1 to 7 of 7

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.
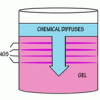
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Exploring Properties: Surface Area
Source Institutions
This hands-on activity demonstrates how a material can act differently when it's nanometer-sized.
