Search Results
Showing results 1 to 20 of 24

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.

Luminol Test
Source Institutions
Learners mix a solution containing luminol and copper with a fake blood solution. A chemical reaction between the luminol solution and fake blood (hydrogen peroxide) show learners a blue glow.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.

Twisted Tesselations
Source Institutions
In this activity (on pages 41-47 of PDF), learners explore tesselating geometric patterns (repeated shapes, similar to the art of M.C. Escher).

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

What's Your Blood Type?
Source Institutions
In this activity, learners perform a simulated blood test procedure.
Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
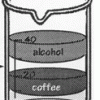
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

Acid Rain
Source Institutions
In this chemistry demonstration, acid rain is simulated in a petri dish.

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Rock Bottoms
Source Institutions
Learners add acid rain (nitric acid) to two cups that represent lakes. One cup contains limestone gravel and the other contains granite gravel.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.
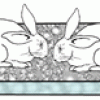
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

History of Electricity
Source Institutions
This is a series of demonstrations about different electrical and magnetic phenomena.
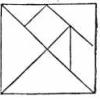
Tricky Tangrams
Source Institutions
In this activity (on pages 49-54 of PDF), learners play with tangrams, a set of triangles, squares and a parallelogram that can combine into a larger square as well as all sorts of other shapes.

