Search Results
Showing results 1 to 20 of 32
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Making With Microbes
Source Institutions
In this design challenge, learners will use microbes to design and grow a custom biomaterial at home and make something creative with it.

Homemade Hovercraft!
Source Institutions
This activity (on page 2 of the PDF under SciGirls Activity: Hovercraft) is a full inquiry investigation into hovercraft engineering and design optimization.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Kites
Source Institutions
This activity (on page 2 of the PDF under SciGirls Activity: Kites) is a full inquiry investigation into how a kite’s shape affects its performance.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.

DNA Nanotechnology
Source Institutions
In this activity, learners explore deoxyribonucleic acid (DNA), a nanoscale structure that occurs in nature.
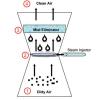
Washing Air
Learners observe and discuss a simple model of a wet scrubber, a device for cleaning industrial air pollution.

Forms of Carbon
Source Institutions
In this activity, educators can demonstrate how the nanoscale arrangement of atoms dramatically impacts a material’s macroscale behavior.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
Properties of Metals
Source Institutions
In this activity, learners explore the properties of metals at four stations. The stations include A) Magnetism and Breakfast Cereal; B) Conductivity of Metals; C) Alloys; and D) Metal Plating.

OBIS Oil Spill
Source Institutions
In this outdoor activity, learners simulate an oil spill using popcorn (both oil and popcorn float on water), and estimate the spill's impact on the environment.
Shrinkers
Source Institutions
Visitors use heat to shrink samples of polystyrene. They compare samples from containers that were shaped in different ways during manufacturing.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.

Vocal Visualizer
Source Institutions
With a bit of PVC, a laser, a can/cup, and a small mirror, you can make a device that visualizes you voice or any sound transmitted into the cup/can.
