Search Results
Showing results 1 to 8 of 8

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Electroplating
Source Institutions
In this electrochemistry activity, learners will explore two examples of electroplating.

Copper Caper
Source Institutions
In this activity, learners conduct an oxidation experiment that turns old pennies bright and shiny. Learners soak 20 dull, dirty pennies in a bowl of salt and vinegar for five minutes.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.
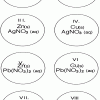
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

What's in a Penny?
Source Institutions
In this chemistry activity, learners use chemical reactions to observe the composition of an alloy.
Physical Change
Source Institutions
In this activity, learners use heat to separate zinc and copper in a penny. This experiment demonstrates physical properties and how physical change (phase change) can be used to separate matter.

Chemical Change
Source Institutions
In this chemistry activity, learners explore the amount of copper in a new penny. Learners use toilet bowl cleaner to hollow out the interior of a penny with zinc inside.
