Search Results
Showing results 1 to 15 of 15

A Little Drop of Water: Cohesion
Source Institutions
Learners explore water's property of cohesion through two investigations.
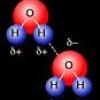
Cohesion Coin
Source Institutions
In this activity about the property of water (page 6 of the PDF), learners use a coin to demonstrate cohesion between water molecules, exploring the molecular forces that allow water molecules to "

Disappearing Colors
Source Institutions
In this challenge, learners figure out how to make a juice stain disappear.

The Colors of Flowers
Source Institutions
In this activity, learners perform an experiment to find out what determines a flower's color.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

Water Quality and pH Levels in Aquatic Ecosystems
Source Institutions
In this fun and in depth hands-on experiment, learners test various liquid samples (distilled water, lemon juice, vinegar, and baking soda mixed with water) to determine their pH levels and identify e
Gas Producing Micro-Reaction
Source Institutions
In this chemistry activity, learners use common chemicals and metals to examine reactions that produce gaseous substances.

Production of Hydrogen
Source Institutions
In this chemistry activity, learners use mossy zinc (or a galvanized nail) and hydrochloric acid to generate hydrogen gas and test some of its properties.
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.

Nuclear Fusion
Source Institutions
This simple and engaging astronomy activity explains nuclear fusion and how radiation is generated by stars, using marshmallows as a model.
