Search Results
Showing results 1 to 9 of 9

Invisible Ink Demonstration
Source Institutions
In this chemistry demonstration, learners will discover that phenolphthalein is a chemical that displays different colors depending on the acidity or basicity of the environment.

The Colors of Flowers
Source Institutions
In this activity, learners perform an experiment to find out what determines a flower's color.

pHun with Cabbage
Source Institutions
In this chemistry activity, learners will test the pH of various foods and household substances using cabbage.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Witches' Potion Demonstration
Source Institutions
In this chemistry demonstration, learners will discover that phenolphthalein is an acid/base indicator. One learner will read a poem about four witches making a potion.

Acids & Bases
Source Institutions
In this activity, learners test the pH of safe liquids available at home by creating a pH indicator from mashed blueberries.

Spicy Indicator: Use turmeric to test for bases in your home
Source Institutions
This activity uses turmeric, a common spice in curry, as an indicator for acidity and basicity. Turmeric is yellow in acid and neutral substances, but turns bright red with bases.
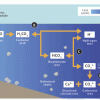
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.

Cabbage Indicator
Source Institutions
In this fun chemistry activity (page 3 of the pdf), learners use cabbage juice to determine the pH of several substances.
