Search Results
Showing results 1 to 9 of 9

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.
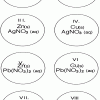
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.

Exploring Properties: Surface Area
Source Institutions
This hands-on activity demonstrates how a material can act differently when it's nanometer-sized.
