Search Results
Showing results 1 to 6 of 6

Electroplating
Source Institutions
In this electrochemistry activity, learners will explore two examples of electroplating.
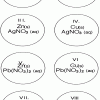
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

What's in a Penny?
Source Institutions
In this chemistry activity, learners use chemical reactions to observe the composition of an alloy.
Physical Change
Source Institutions
In this activity, learners use heat to separate zinc and copper in a penny. This experiment demonstrates physical properties and how physical change (phase change) can be used to separate matter.

Science of Sunblock
Source Institutions
This is an activity (located on page 3 of the PDF under Stained Glass Activity) about nanotechnology making its way into everyday products, such as sunscreen, and how effective these invisible particl

Chemical Change
Source Institutions
In this chemistry activity, learners explore the amount of copper in a new penny. Learners use toilet bowl cleaner to hollow out the interior of a penny with zinc inside.
