Search Results
Showing results 1 to 19 of 19
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.

Investigating Starch
Source Institutions
In this activity (on pages 10-15), learners investigate starch in human diets and how plants make starch (carbohydrates) to use as their food source.

Law of Conservation of Mass
Source Institutions
In this chemistry activity, learners explore whether matter is created or destroyed during a chemical reaction. They will compare the weight of various solutions before and after they are mixed.

Fruit Juice Mystery
Source Institutions
In this chemistry challenge, learners work to figure out which of four juices are real, and which is just food coloring and sugar.

How Greenhouse Gases Absorb Heat
Source Institutions
Learners observe two model atmospheres -- one with normal atmospheric composition and another with an elevated concentration of carbon dioxide.

Starch Breakdown
Source Institutions
Learners use Benedict’s solution and heat to test for the presence of simple sugars in glucose, sucrose, starch, and starch combined with amylase.
Make Your Own DNA
Source Institutions
Learners match puzzle pieces to outlines of a DNA strand. The puzzle pieces represent the four chemicals making up DNA base pairs: adenine, thymine, guanine, and cytosine.

Cool It
Source Institutions
In this outdoor activity/game, learners use thermometers to simulate how lizards survive in habitats with extreme temperatures.

Cooking With the Sun
Source Institutions
In this activity, learners build a simple solar oven out of household materials to melt chocolate and marshmallow between graham crackers--known as s'mores.

Why Does Food Spoil?
Source Institutions
In this activity, learners will conduct an experiment to discover methods of reventing foot mold growth on food.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.
Stability of Egg White Foams
Source Institutions
In this chemistry meets cooking activity, learners compare the stability of egg white foams with various additives.

Whodunit?
Source Institutions
In this fascinating and fun experiment, learners use chemistry to identify a mystery powder and to solve a "crime," a process similar to that used by real forensic scientists.
Spectroscope
Source Institutions
In this activity, learners construct their own spectroscope as they explore and observe spectra from familiar light sources.

Fireworks!
Source Institutions
In this chemistry lab activity, learners model the colors of fireworks by burning metallic solutions in a flame and observing the different colors produced.

First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.
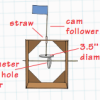
Crank It Up
Source Institutions
In this engineering activity, learners explore simple machines and then build cardboard automata using cams.

Photosynthetic Pictures Are Worth More Than a Thousand Words
Source Institutions
This activity provides an opportunity for learners to observe and examine how carbon dioxide, water, and light produce glucose/starch through a process called photosynthesis.

Blimp Jet Challenge
Source Institutions
In this design challenge activity, learners add a jet-propulsion system (i.e. a balloon) to a blimp so it flies straight and far under its own power.
