Search Results
Showing results 41 to 60 of 93
Make Your Own DNA
Source Institutions
Learners match puzzle pieces to outlines of a DNA strand. The puzzle pieces represent the four chemicals making up DNA base pairs: adenine, thymine, guanine, and cytosine.

Changing Colors
Source Institutions
In this challenge, learners have to figure out in what order to combine five solutions to change the color from clear, to yellow, to blue, and back to clear.

Magic Inks
Source Institutions
Learners write their initials by applying different clear "magic ink" solutions to separate pieces of paper and then "develop" the inks with other clear solutions.
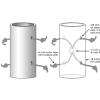
Mystery Tubes
Source Institutions
Learners investigate a pre-constructed mystery tube to determine its interior mechanism.

Jumpin' the Gap
Source Institutions
In this simulation of synapses, learners act out communication at the neural level by behaving as pre-synaptic vesicles, neurotransmitters, post-synaptic receptors, secondary messengers and re-uptake

Cool It
Source Institutions
In this outdoor activity/game, learners use thermometers to simulate how lizards survive in habitats with extreme temperatures.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.

Vocal Visualizer
Source Institutions
With a bit of PVC, a laser, a can/cup, and a small mirror, you can make a device that visualizes you voice or any sound transmitted into the cup/can.

Off Base
Source Institutions
In this activity, learners explore the factors that tend to resist changes in pH of the ocean and why the ocean is becoming more acidic.

Recycling Rules: Understanding Recycling and a MRF
Source Institutions
In this activity, learners simulate the separation techniques that materials recovery facilities (MRFs) use and then design their own series of recycling techniques.
Great Steamboat Race
Source Institutions
In this outdoor activity, learners race small boats, made of cork, balsa wood, popsicle sticks etc., to investigate the rate and direction of currents in a stream or creek.

Electroplating
Source Institutions
In this activity, learners electrically plate zinc onto brass objects.

Thar She Glows!
Source Institutions
Learners observe glow-in-the-dark objects in a homemade light-proof box. Objects can include glow sticks, glow-in-the-dark toys, and toys with fluorescent paint.

Cooking With the Sun
Source Institutions
In this activity, learners build a simple solar oven out of household materials to melt chocolate and marshmallow between graham crackers--known as s'mores.

The Carbon Cycle and its Role in Climate Change: Activity 1
Source Institutions
In this activity (on page 1), learners role play as atoms to explore how atoms can be rearranged to make different materials.

Why Does Food Spoil?
Source Institutions
In this activity, learners will conduct an experiment to discover methods of reventing foot mold growth on food.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.
Stability of Egg White Foams
Source Institutions
In this chemistry meets cooking activity, learners compare the stability of egg white foams with various additives.
