Search Results
Showing results 1 to 20 of 23

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Release the Grease!
Source Institutions
In this simple activity (on page 7 of the PDF), learners use water and liquid dish detergent to see which one removes lipstick better from an index card.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Bubble Trouble
Source Institutions
In this activity on page 15 of the PDF, learners measure the amount of bubbles that they make using a detergent.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.
Making Recycled Paper
Source Institutions
In this activity on page 11 of the PDF, learners follow simple steps to recycle old newspaper into new paper.
Another Bright Idea!
Source Institutions
In this activity on page 5 of the PDF, learners use their knowledge of energy and batteries to create homemade flashlights.

Secret Goldenrod Messages
Source Institutions
In this activity, learners write invisible messages on goldenrod paper, and make the message appear and disappear using acids and bases.

Spaghetti Strength
Source Institutions
In this activity on page 7 of the PDF, learners explore how engineers characterize building materials.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

What Counts in Bounce
Source Institutions
In this activity learners compare the bounciness of warm and cold racquetballs to see if temperature makes a difference in how well they bounce.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Special Effects Using Household Chemicals
Source Institutions
In this activity on page 4 of the PDF (Behind the Scenes with Chemistry), learners make some special effects, including snow and breaking glass, with supplies found in the home.

Safe in the Sun
Source Institutions
In this activity on page 13 of the PDF, use a special plastic card that has been painted with a chemical that changes color when it is in UV light.
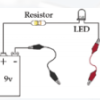
Exploring Materials: Graphene
Source Institutions
In this activity on page 4 of the PDF, explore the unique molecular structure and conductive nature of graphene. Learners construct a circuit with a battery and LED bulb.

Homework, Hogwarts Style
Source Institutions
In this activity on page 8 of the PDF (Behind the Scenes with Chemistry), learners make three of Harry Potter's essential school supplies: quills, ink, and color-changing paper.
