Search Results
Showing results 61 to 80 of 156
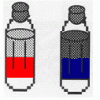
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.
Bring it into Focus
Source Institutions
In this activity (page 2 of PDF), learners play with a lens and a piece of paper to focus an image on the paper. Learners look at different things, and see how the lenses affect the image.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.
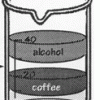
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Recycling Paper
Source Institutions
In this crafty chemistry activity (on page 2 of the PDF), learners make their own paper from used paper they may have otherwise thrown away.
Roller Ball Painting
Source Institutions
This is an activity in which learners explore the effects of gravity, motion and momentum while creating art.
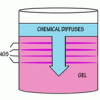
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

Electroplating
Source Institutions
In this activity, learners electrically plate zinc onto brass objects.

Resistance is Useful
Source Institutions
Learners write or draw with white crayon on white paper. They look and feel to detect their marks on the paper. Then, learners paint over their paper with watercolor paint.

Water Ways
Source Institutions
In this activity (on page 2 of the PDF), learners explore surface tension by adding pennies to cups which are "full" of plain water or soapy water.

Pearlescent Pigments
Source Institutions
This is written as a display, but can easily be adapted to a hands-on activity. Learners observe and shake containers of shiny liquids.

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).

Rainbow Glasses
Source Institutions
In this activity, learners explore light, color and rainbows by making their own rainbow glasses.

Salt Painting
Source Institutions
In this art meets chemistry activity, early learners discover the almost magical absorbent properties of salt while creating ethereal watercolor paintings.

Thar She Glows!
Source Institutions
Learners observe glow-in-the-dark objects in a homemade light-proof box. Objects can include glow sticks, glow-in-the-dark toys, and toys with fluorescent paint.

