Search Results
Showing results 1 to 20 of 46

Balloon Isometrics
Source Institutions
In this chemistry activity, learners will explore the concept of entropy. When learners stretch and unstretch a balloon, they will notice a change in temperature.

How Can Gravity Make Something Go Up?
Source Institutions
In this activity, learners use cheap, thin plastic garbage bags to quickly build a solar hot air balloon. In doing so, learners will explore why hot air rises.

Earth's Energy Cycle: Albedo
Source Institutions
In this activity, learners experiment and observe how the color of materials that cover the Earth affects the amounts of sunlight our planet absorbs.

Hot and Cold
Source Institutions
In this chemistry challenge, learners discover that many chemical reactions involve heat loss or gain.

Nano Ice Cream
Source Institutions
In this activity/demo, learners discover how liquid nitrogen cools a creamy mixture at such a rapid rate that it precipitates super fine grained (nano) ice cream.
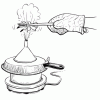
Going for a Spin: Making a Model Steam Turbine
Source Institutions
In this activity, learners explore how various energy sources can be used to cause a turbine to rotate.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Understanding Albedo
Source Institutions
In this activity related to climate change, learners examine albedo and the ice albedo feedback effect as it relates to snow, ice, and the likely results of reduced snow and ice cover on global temper

Fog Chamber
Source Institutions
In this weather-related activity, learners make a portable cloud in a bottle.

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.

Meltdown
Source Institutions
In this activity, learners heat ice and water of the same temperature to get a hands-on look at phase changes. This is an easy and inexpensive way to introduce states of matter and thermodynamics.

Solar Energy
Source Institutions
In this activity (page 11 of PDF), learners compare the air pressure within a dark and a light bottle both heated by the sun, and discover that solar energy can be collected and stored in many ways

Solar Water Heater
Learners work in teams to design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy.

Lifting Lemon
Source Institutions
In this physics demonstration, learners will be surprised when a lemon slice appears to magically levitate within a pint glass.
Sodium Acetate Hand Warmers
Source Institutions
In this activity, sodium acetate hand warmers are used to introduce learners to supersaturated solutions, crystallization, and exothermic reactions.

Balloon Inside a Bottle
Source Institutions
In this activity about phase change and condensation, learners boil water in an empty pop bottle in the microwave.
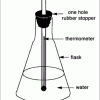
Using Solar Energy
Source Institutions
In this activity, learners discover how solar energy can be used to heat water.

Geothermal Power Plant Model
Source Institutions
In this activity, learners make a model of a power plant that uses steam. Learners use simple materials like foil, a tin can, and a pot of water to model a geothermal power plant.

What Counts in Bounce
Source Institutions
In this activity learners compare the bounciness of warm and cold racquetballs to see if temperature makes a difference in how well they bounce.

Thermal Energy Put to Work
Source Institutions
In this activity, learners determine whether thermal energy can be used for work.
