Search Results
Showing results 221 to 240 of 799

The Carbon Cycle: How It Works
Source Institutions
In this game, learners walk through an imaginary Carbon Cycle and explore the ways in which carbon is stored in reservoirs and the processes that transport the carbon atom from one location to another

That Sinking Feeling
Source Institutions
In this quick activity, learners observe how salinity and temperature affect the density of water, to better understand the Great Ocean Conveyor.

Curious Crystals
Source Institutions
Learners carefully look at four known household crystals.

Color Me Blue
Source Institutions
In this activity, learners add dilute bleach solution to water that has been dyed with yellow, blue, and green food color.
Daffy Density
Source Institutions
In this chemistry activity, learners explore density by using four solids and 6 liquids to create colorful, layered rows.
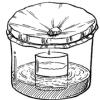
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.

Finding Red
Source Institutions
In this chemistry challenge, learners systematically investigate which combination of four solutions produces a deep red color.

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.

Dinosaur Breath
Through discussion and hands-on experimentation, learners examine the geological (ancient) carbon cycle.

Kool Colors
Source Institutions
Learn about dyes and mordants (fixatives) when you tie-dye fabric with Kool-Aid™ and vinegar. The colored molecules in Kool-Aid™ form a chemical bond between the fiber and dye molecules.

Moving Without Wheels
In a class demonstration, learners observe a simple water cycle model to better understand its role in pollutant transport.

Silver Crystals
Source Institutions
This is written as a static display, but can easily become a hands-on experiment for learners.

Gelatin Used for Drug Delivery
Source Institutions
In this activity, learners discover how gelatin can be used as a medium for drug delivery. Learners create colored gelatin and then cut out pieces of the gelatin to simulate medicine (pills).

Hot Stuff!: Carbon Dioxide Extinguishes a Flame
In this demonstration, learners observe vinegar and baking soda creating carbon dioxide (CO2) in a bottle. The gas is poured out of a bottle onto a candle flame, putting out the candle.

Diatom Ooze: Ooze Clues
Source Institutions
In this activity, learners will plot the distribution of various oozes using information from sediment maps.

Law of Conservation of Mass
Source Institutions
In this chemistry activity, learners explore whether matter is created or destroyed during a chemical reaction. They will compare the weight of various solutions before and after they are mixed.

How Long Can You Hold Your Breath?
Source Institutions
In this activity (on page 142 of the PDF), learners will compare breathing rates before and after hyperventilation to explore how reduced carbon dioxide levels in the blood lower the need to breathe.

Of Cabbages and Kings
Source Institutions
This lesson gives full instructions for making cabbage juice indicator, a procedure sheet for learners to record observations as they use the indicator to test materials, and extension activities to d

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.
