Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
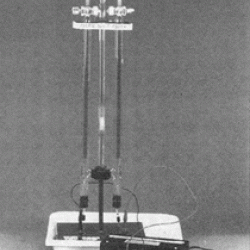
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water. Oxygen gas is formed in the tube connected to the positive electrode. Hydrogen gas is produced in the tube connected to the negative electrode. As the gases are formed, they float to the top where they can be collected for demonstrations: 1) a hydrogen-filled tube will make an exploding sound when lit; 2) an oxygen-filled test tube will re-light a glowing splint; and 3) a hydrogen- and oxygen-filled rocket can be launched across the room. This is an exhibit and demonstration in OMSI's Chemistry Lab and would make a good demonstration for classroom students or other learners.
- 1 to 2 hours
- Under 5 minutes
- $10 - $20 per group of students
- Ages 11 - adult
- Activity, Demonstration, Exhibit
- English
Quick Guide
Materials List (per group of students)
- Hoffman electrolysis apparatus (WARDS Biology/lab Supplies, 1-800-962-2660)
- Bobbitt low-Voltage (12V DC) Power Supply (Carolina Science and Math, 1-800-227-1150)
- Concentrated H2SO4 (sulfuric acid) (keep 100 ml on hand)
- Two clean, dry, medium-sized test tubes
- 10-ml graduated cylinder
- 600-ml beaker
- Deionized water (dH2O) (keep 500 ml on hand)
- Wooden splints
- Candle
- Butane lighter or matches
Subjects
-
Physical Sciences
-
Electricity and Magnetism
- Electric Charges and Currents
-
Chemistry
- Chemical Reactions
-
States of Matter
- Gases
-
Electricity and Magnetism
Audience
To use this activity, learners need to:
- see
- see color
- hear
Learning styles supported:
- Involves hands-on or lab activities
Other
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, Oregon Museum of Science and Industry, 1997
Funding Source:
- National Science Foundation
