Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
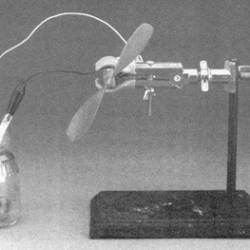
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan. Learners observe that chemical reactions can produce electricity. This is a one-cell battery, so only creates a small amount of power. Batteries are usually collections of many cells to produce greater amounts of power. This is written as a display, but can easily be adapted for learners to build their own electrochemical reactions.
- 10 to 30 minutes
- 10 to 30 minutes
- $5 - $10 per group of students
- Ages 11 - adult
- Activity, Experiment/Lab Activity
- English
Quick Guide
Materials List (per group of students)
- Small baby-food jars (or small beakers) (keep five on hand)
- One-holed stoppers (size 7) (keep five on hand)
- Zinc foil strips 6 in. long (15 cm) (keep several on hand)
- Copper foil strips 6 in. long (15 cm) (keep several on hand)
- One spool of dialysis tubing
- Wire leads with alligator clips
- One small motor/fan, similar to those in toy construction sets
- One clamp and ringstand
- 1.0M CuSO4 (copper sulfate) solution (500 ml reserve)
- 0.5M Na2SO4 (sodium sulfate) solution (500 ml reserve)
- Steel wool (keep three to four pieces on hand)
- Plastic pipette
- Resealable plastic sandwich bags (keep four boxes on hand)
- small DC motor
- fan
Subjects
-
Physical Sciences
-
Electricity and Magnetism
- Electric Charges and Currents
-
Chemistry
- Chemical Reactions
- Oxidation-Reduction Reactions
-
Electricity and Magnetism
Audience
To use this activity, learners need to:
- see
- read
Learning styles supported:
- Involves hands-on or lab activities
Other
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, Oregon Museum of Science and Industry, 1997
Funding Source:
- National Science Foundation
