Source Institutions
Source Institutions
Add to list Go to activity
Activity link broken? See if it's at the internet archive
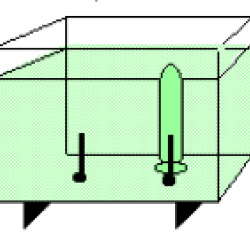
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution. A pH indicator (bromthymol blue) in the water allows learners to observe how the pH of the solution changes as the reaction proceeds. Background information explains the reaction, and also how industry uses electricity to separate chemical compounds such as sodium chloride (table salt) into chlorine gas and pure sodium metal.
- 30 to 45 minutes
- Under 5 minutes
- $10 - $20 per group of students
- Ages 6 - adult
- Activity, Experiment/Lab Activity
- English
Quick Guide
Materials List (per group of students)
- One plastic electrolysis tub (from Central Scientific catalog: “compact electrolysis apparatus”)
- Two small (13- by 100-mm) test tubes (keep four on hand)
- One plastic pipette (keep two on hand)
- One wooden stirring stick (keep six on hand)
- Two 6V lantern batteries (keep two fresh batteries on hand)
- Two wire leads (each 8 to 12 inches long) with alligator clips on each end (one red wire, one white wire)
- Two black leads (each 8 to 12 inches long) with an alligator clip on one end only
- Three 30-ml dropper bottles
- 0.1M HCl (hydrochloric acid) (keep 100 ml on hand) OR — 1M HCl (keep 1 l on hand) OR — concentrated (12M) HCl (keep 100 ml on hand)
- 0.1M NaOH (sodium hydroxide) (keep 100 ml on hand) OR — 1M NaOH (sodium hydroxide) (keep 25 ml on hand) OR — solid NaOH (keep 500 g on hand)
- Two 125-ml plastic storage bottles
- One 1000-ml plastic storage bottle
- Na2SO4*10H2O (sodium sulfate) (keep 250 g on hand)
- Bromthymol blue (keep 25 ml on hand)
- One knife switch
- Two small screws
- One wooden block approximately 3 inches wide and 8 inches long
- One 600-ml beaker or flask
Subjects
-
Engineering and Technology
-
Engineering
- Chemical Engineering
- Technology
-
Engineering
-
Physical Sciences
- Electricity and Magnetism
-
Chemistry
- Acids and Bases
- Oxidation-Reduction Reactions
- Solutions
- Structure and Properties of Matter
- The Nature of Technology
Audience
To use this activity, learners need to:
- see
- see color
- read
- touch
Learning styles supported:
- Involves hands-on or lab activities
Other
Components that are part of this resource:
This resource is part of:
Access Rights:
- Free access
By:
Rights:
- All rights reserved, Oregon Museum of Science and Industry, 1997
Funding Source:
- National Science Foundation
