Search Results
Showing results 21 to 40 of 55

Space Jell-O
Source Institutions
Albert Einstein proved that space bends around anything that has mass. This activity uses Jell-O's ability to bend around objects as a model for space bending around planets and stars.
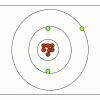
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Ice Cream Freeze
Source Institutions
In this fun and delicious chemistry activity (page 1 of the PDF), learners will explore the difference between physical and chemical change by making homemade ice cream.

Conduction Countdown
Source Institutions
In this quick SciGirls activity (page 1 of the PDF under SciGirls Activity: Doghouse Design), learners will be introduced to the concept of thermal conductivity.

Solar Powered Cooking
Source Institutions
In this activity, learners make a solar oven. Learners witness the awesome power of the sun to make a yummy treat--a chocolate chip cookie!

Investigating the Line
Source Institutions
In the related activity called "Colors Collide or Combine," learners are intrigued by the apparent "line" that forms where colors from M&M coatings meet but do not mix.
Stability of Egg White Foams
Source Institutions
In this chemistry meets cooking activity, learners compare the stability of egg white foams with various additives.

Wrap It Up!
Source Institutions
In this Energy and Environment activity (page 9 of the PDF), learners calculate the mass of a piece of gum, compare it to the mass of the gum's packaging, and then create a bar graph of the results.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.

Jiggly Jupiter
Source Institutions
In this activity, learners build edible models of Jupiter and Earth to compare their sizes and illustrate the planets' internal layers.

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.

Bridge the Gap
Source Institutions
Learners work in groups to construct bridges using stale marshmallows and toothpicks.

Milk Plastic
Source Institutions
In this activity, learners transform everyday milk into small plastic figurines and jewelry. Use this activity to introduce learners to monomers and polymers.

Kosher Dill Current: Make Your Own Battery!
Source Institutions
This is an activity that demonstrates how batteries work using simple household materials. Learners use a pickle, aluminum foil and a pencil to create an electrical circuit that powers a buzzer.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.
Build a Super Structure
Source Institutions
In this activity, learners use things from the kitchen as building materials to explore how shapes contribute to the strength of different structures.

Molecular Menagerie
Source Institutions
In this activity, learners use molecular model kits to construct familiar molecules like lactose, caffeine, and Aspirin.
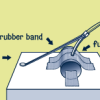
Target Practice
Source Institutions
In this dynamic activity, learners build a catapult that launches projectiles, such as marshmallows.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.

Do the Mystery Samples Contain Life?
Source Institutions
In this activity (on pages 13-16 of the PDF) learners investigate three mystery samples to see which one contains life. The three samples are sand, sand and yeast, and sand and antacid.
