Search Results
Showing results 1 to 20 of 28

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Jelly Beads
Source Institutions
Learners add drops of alginate solution to a solution of calcium chloride. The alginate does not mix with the calcium chloride, but forms soft gel beads.

Bendy Bones
Source Institutions
In this activity (on pages 19-24 of PDF), learners soak chicken bones or eggshells in vinegar for several days.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.
Busted by Biology
Source Institutions
In this two-part activity, learners will extract their own DNA from their cheek cells and learn how DNA is analyzed and used to solve crimes.
Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Egg Osmosis: A four day eggsperience!
Source Institutions
Eggs are placed in vinegar for one or two days to dissolve the shells. Then, learners place the eggs in water or corn syrup and observe them over a period of days.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.
Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.

Spicy Indicator: Use turmeric to test for bases in your home
Source Institutions
This activity uses turmeric, a common spice in curry, as an indicator for acidity and basicity. Turmeric is yellow in acid and neutral substances, but turns bright red with bases.
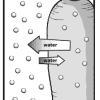
Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Natural Indicators
Source Institutions
Learners combine different plant solutions -- made from fruits, vegetables, and flowers -- with equal amounts of vinegar (acid), water (neutral), and ammonia (base).

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.

Kool Colors
Source Institutions
Learners investigate how temperature affects the rate of chemical reactions by observing how steel wool reacts with various types of Kool-Aid solutions at different temperatures.

