Search Results
Showing results 201 to 220 of 277

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

Molecular Menagerie
Source Institutions
In this activity, learners use molecular model kits to construct familiar molecules like lactose, caffeine, and Aspirin.

Kimchee Fermentation Chamber
Source Institutions
Learners make kimchee or sauerkraut, which is really just fermented cabbage, in a 2-liter plastic bottle.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.

Testing Vitamin C: Chemistry's Clear Solution
Source Institutions
In this activity on page 8 of the PDF, learners investigate vitamin C. Learners conduct a chemistry experiment to determine if Tang drink mix or orange juice contains more vitamin C.

Bubble Bomb
Source Institutions
Learn about chemical reactions by making a Bubble Bomb, a plastic bag you can pop with the power of fizz.

Enzyme Action
Source Institutions
In this activity that can be used as a lab or demonstration, learners use Lactaid® and lactose to demonstrate the concept of enzyme action.

Temperature vs. Height: Soda Geyser Series #6
Source Institutions
In this activity, learners conduct a controlled experiment to examine how temperature will affect the height of a soda geyser.

Spicy Indicator: Use turmeric to test for bases in your home
Source Institutions
This activity uses turmeric, a common spice in curry, as an indicator for acidity and basicity. Turmeric is yellow in acid and neutral substances, but turns bright red with bases.

Do the Mystery Samples Contain Life?
Source Institutions
In this activity (on pages 13-16 of the PDF) learners investigate three mystery samples to see which one contains life. The three samples are sand, sand and yeast, and sand and antacid.

Fruity Electricity
Source Institutions
In this activity, Frankenstein's lab is running out of electricity! Learners use fruit to help Igor find a temporary source of energy to turn on a light.
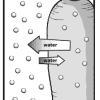
Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Thymus DNA Extractions
Source Institutions
This laboratory exercise is designed to show learners how DNA can be extracted from a chunk of thymus (sweetbread) or liver.

Soda Brand vs. Height Experiment: Soda Geyser Series #4
Source Institutions
In this activity, learners conduct a controlled experiment to examine which brand of soda makes the best (highest) soda geyser.

Say Cheese!
Source Institutions
Create a chemical reaction that makes cheese! This hands-on activity demonstrates that molecules and atoms are tiny particles that make up everything around us.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Gummy Shapes
Source Institutions
In this activity, learners use chemistry to “self-assemble” gummy shapes. Learners discover that self-assembly is a process by which molecules and cells form themselves into functional structures.

Radioactive Decay of Candium
Source Institutions
In this simulation, learners use M&M™ candy to explore radioactive isotope decay.

Erupting Fizz
Source Institutions
This is a highly visual demonstration that illustrates both the effects of density and chemical reactions.

Copper Cleanup
Source Institutions
In this hands-on experiment, kids use chemistry to explore whether acids or bases are better at restoring a penny’s shine.
