Search Results
Showing results 1 to 20 of 22

Sweet Measurements
Source Institutions
In this activity on page 3 of the PDF, learners investigate how much sugar is in a soda. Learners use sugar cubes to measure and calculate the amount of sugar in a bottle of soda.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.

Fruity Electricity
Source Institutions
In this activity, Frankenstein's lab is running out of electricity! Learners use fruit to help Igor find a temporary source of energy to turn on a light.

Turbidity
Source Institutions
This is an activity about turbidity, or the amount of sediment suspended in water.

Change in Temperature: Exothermic Reaction
Source Institutions
Learners add calcium chloride to a baking soda solution and observe an increase in temperature along with the production of a gas and a white precipitate. These are all signs of a chemical reaction.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!

Be A Pasta Food Scientist
Source Institutions
In this activity, learners of all ages can become food scientists by experimenting with flour and water to make basic pasta.
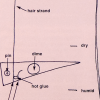
Better Hair Through Chemistry
Source Institutions
In this activity, learners hook up a hair to a lever system and create a hair hygrometer to measure changes in humidity.

A Swell Activity with Beans
Source Institutions
In this combination chemistry and physics activity, learners explore water absorption in dried beans or peas and learn how this affects their physical properties.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Mold Mole Molds
Source Institutions
In this activity, learners make different shapes that hold exactly one mole of gas (air).
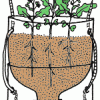
TerrAqua Investigation Column: What is the Land-Water Connection?
Source Institutions
In this investigation, learners plant seeds in a 2-liter bottle filled with soil that is connected to a water source below. Over the next few weeks, learners observe how the plants grow.

Bubble Trouble
Source Institutions
In this activity on page 15 of the PDF, learners measure the amount of bubbles that they make using a detergent.

Conservation of Mass
Source Institutions
This activity was designed for blind learners, but all types of learners can participate to learn about conservation of gas. This is one of the classic experiments using baking soda and vinegar.

Kimchee Fermentation Chamber
Source Institutions
Learners make kimchee or sauerkraut, which is really just fermented cabbage, in a 2-liter plastic bottle.

Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

There's Always Room For JELL-O
Source Institutions
In this activity, learners cut wells in JELL-O© and load the wells with different detergent solutions.

How Big is Small
Source Institutions
In this classic hands-on activity, learners estimate the length of a molecule by floating a fatty acid (oleic acid) on water.

Production of a Gas: Controlling a Chemical Reaction
Source Institutions
Learners mix vinegar and baking soda to produce a gas. With the addition of a bit of liquid soap, the gas becomes trapped in measurable bubbles.

Chromatography Can Separate!
Source Institutions
In this chemistry activity, learners use thin layer chromatography to determine the molecular composition of different markers.
