Search Results
Showing results 1 to 14 of 14

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.
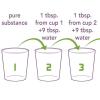
Sniffing for a Billionth
Source Institutions
This is an activity (located on page 4 of the PDF under What's Nano? Activity) about size and scale.

Gravity Fail
Source Institutions
In this activity, learners try pouring water out of a regular cup and a miniature cup. It’s harder than it sounds! Learners discover that different forces dominate at different size scales.

Hot Stuff!: Carbon Dioxide Extinguishes a Flame
In this demonstration, learners observe vinegar and baking soda creating carbon dioxide (CO2) in a bottle. The gas is poured out of a bottle onto a candle flame, putting out the candle.

Hot Stuff!: Testing for Carbon Dioxide from Our Own Breath
Learners blow into balloons and collect their breath--carbon dioxide gas (CO2). They then blow the CO2 from the balloon into a solution of acid-base indicator.

Try Your Hand at Nano
Source Institutions
This lesson focuses on two simple activities that younger learners can do to gain an appreciation of nanotechnology. First, learners measure their hands in nanometers.

Shrinking Cups
Source Institutions
This is a quick activity (on page 2 of the PDF under Gecko Feet Activity) about the forces of gravity and surface tension and how their behavior is influenced by size.

Acid Rain Effects
Learners conduct a simple experiment to model and explore the harmful effects of acid rain (vinegar) on living (green leaf and eggshell) and non-living (paper clip) objects.

Hot Stuff!: Testing Ice
In this demonstration, learners compare and contrast regular water ice to dry ice (frozen carbon dioxide). Both samples are placed in a solution of acid-base indicator.

Cabbage Juice Indicator
Source Institutions
In this chemistry activity, learners make indicator solution from red cabbage. Then, learners test everyday foods and household substances using the cabbage juice indicator.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Fizzy Nano Challenge
Source Institutions
This lesson focuses on how materials behave differently as their surface area increases.
