Search Results
Showing results 1 to 20 of 56

Levity Through Tension: Fun with Water's Surface Tension
Source Institutions
This experiment describes how to create a "dribble bottle" which only leaks water when the cap is unscrewed. The full water bottle has a small hole made with a push pin.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Drop Shape
Source Institutions
In this activity, learners get a closer look at the shape of a drop of water and a drop of oil. Learners first drip water onto wax paper and examine the shape of separate drops from a side view.

Penny Drop
Source Institutions
In this quick activity about the properties of water (page 1 of PDF under SciGirls Activity: Malformed Frogs), learners will use an eyedropper to slowly place one drop of water at a time onto a penny,
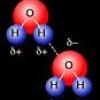
Cohesion Coin
Source Institutions
In this activity about the property of water (page 6 of the PDF), learners use a coin to demonstrate cohesion between water molecules, exploring the molecular forces that allow water molecules to "

Frosty Glasses
Source Institutions
In this activity, learners explore why frost forms. They create their own frost using a solution of ice water and salt in a glass.

Changing the Density of a Liquid: Adding Salt
Source Institutions
Learners see that a carrot slice sinks in fresh water and floats in saltwater.

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.

Gravity-Defying Water
Source Institutions
In this activity, learners explore gravity and air pressure as they experiment with holding a glass full of water upside down, without spilling it, using a simple piece of cardstock.
Floating Paperclip and Other Surface Tension Experiments
Source Institutions
In this activity, learners experiment with surface tension using everyday household items such as strawberry baskets, paperclips, liquid dish soap, and pepper.

How Much Water is in that Cloud?
Source Institutions
In this activity, learners working in pairs saturate a cotton ball using water drops from an eyedropper to demonstrate the high water capacity of clouds.

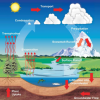
The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.
Turning the Air Upside Down: Convection Current Model
Learners see convection currents in action in this highly visual demonstration. Sealed bags of colored hot or cold water are immersed in tanks of water.

Design a Submarine
Source Institutions
Learners act as engineers and design mini submarines that move in the water like real submarines.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Ocean in a Bottle
Source Institutions
In this simulation activity, learners observe what can happen when ocean waves churn up water and oil from an oil spill.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.
Pollution in Our Watershed
Source Institutions
By building a simple watershed with paper and markers and then using a spray bottle to simulate precipitation, learners will understand how pollution accumulates in our water sources, especially from

Fog Chamber
Source Institutions
In this weather-related activity, learners make a portable cloud in a bottle.
