Search Results
Showing results 21 to 40 of 125
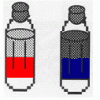
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Dye Detective
Source Institutions
Learners use filter paper and water to analyze six different markers. They mark the paper with ink, and dip the paper in water. The water travels up the paper and dissolved ink travels with it.
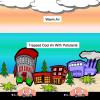
Turning the Air Upside Down: Convection Current Model
Learners see convection currents in action in this highly visual demonstration. Sealed bags of colored hot or cold water are immersed in tanks of water.

Anti-Gravity Cups
Source Institutions
In this activity, learners will use simple materials to explore centripetal force and variables by swinging a cup of water without having the water spill out.

Window Under Water
Source Institutions
Glare from the sun and ripples from the wind can make it hard to see what's below the surface of a body of water.

Let's Look at Water & the Scientific Method
Source Institutions
This activity has learners observe water and compare it to other liquids.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Lighting Up Celery Stalks
Source Institutions
In this activity, learners conduct a series of hands-on experiments that demonstrate how the working of plants' veins, known as capillary action, enables water to travel throughout the length of a pla

Forces at the Nanoscale: Nano Properties of Everyday Plants
Source Institutions
This is an activity (located on page 3 of PDF under Nasturtium Leaves Activity) about surface tension.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Breathing Yeasties
Source Institutions
Does yeast breathe? Find out by watching how plastic bags filled with yeast, warm water and different amounts of sugar change over time.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.
Pollution in Our Watershed
Source Institutions
By building a simple watershed with paper and markers and then using a spray bottle to simulate precipitation, learners will understand how pollution accumulates in our water sources, especially from

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.

Make a UV Detector
Source Institutions
In this activity, learners use tonic water to detect ultraviolet (UV) light from the Sun and explore the concept of fluorescence.

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).
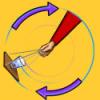
Up and Over
Source Institutions
This is an activity about Newton's First Law of Motion - a body in motion tends to stay in motion, or a body at rest tends to stay at rest unless acted upon by an outside force.

Egg Osmosis: A four day eggsperience!
Source Institutions
Eggs are placed in vinegar for one or two days to dissolve the shells. Then, learners place the eggs in water or corn syrup and observe them over a period of days.

Evaporation
Source Institutions
This three-part activity consists of an activity that groups of learners develop themselves, a given procedure, and an optional demonstration.

Moving Without Wheels
In a class demonstration, learners observe a simple water cycle model to better understand its role in pollutant transport.
