Search Results
Showing results 1 to 20 of 107

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.

Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.
Cool Trees
Source Institutions
This warm weather activity introduces learners to the impact trees have on blocking the sun's heat and reducing temperature on the Earth's surface.

Heat Capacity: Can't Take the Heat?
Source Institutions
Why is ocean water sometimes the warmest when the average daily air temperature starts to drop? In this activity, learners explore the differing heat capacities of water and air using real data.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Differing Densities: Fresh and Salt Water
Source Institutions
In this activity, learners visualize the differences in water density and relate this to the potential consequences of increased glacial melting.

Sinking Water
Source Institutions
In this experiment, learners float colored ice cubes in hot and cold water.

Frozen Sculptures
Source Institutions
In this activity, learners use objects they find on a nature and water to make creative frozen sculptures.

Convection Current
Source Institutions
In this activity, learners make their own heat waves in an aquarium.

Cool It!
Source Institutions
Learners make a refrigerator that works without electricity. The pot-in-pot refrigerator works by evaporation: a layer of sand is placed between two terra cotta pots and thoroughly soaked with water.

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.
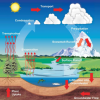
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Do Sweat It!
Source Institutions
In this activity, learners explore why humans sweat. Learners compare the effects of heat on a balloon filled with air and a balloon filled water.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

Why Doesn’t the Ocean Freeze?
Source Institutions
In this activity, learners explore how salt water freezes in comparison to fresh water.
