Search Results
Showing results 21 to 40 of 62

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.

Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.
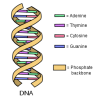
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.

Dip Dip, Hooray
Source Institutions
Lakes, streams and other freshwater bodies are a habitat for lots of living things, big and small.

Make a Lake
Source Institutions
Where rainwater goes after the rain stops? And why there are rivers and lakes in some parts of the land but not in others?

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.

A Crayon Rock Cycle- Metamorphic
Source Institutions
This is part 2 of the three-part "Crayon Rock Cycle" activity and must be done after part 1: Sedimentary Rocks. In this activity, learners explore how metamorphic rocks form.

Glow in the Dark Jello
Source Institutions
In this activity, learners will make homemade jello that glows under a blacklight. They will learn about quinine, an ingredient in tonic water that is fluorescent.

Ice Melt
Source Institutions
In this activity, learners will explore basic information about thermodynamics by experimenting with ice. Learners will compare ice melting rates on metal pans or plastic cutting boards.

Aluminum-Air Battery: Foiled again!
Source Institutions
Construct a simple battery that's able to power a small light or motor out of foil, salt water, and charcoal. A helpful video, produced by the Exploratorium, guides you along on this activity.
Making An Impact!
Source Institutions
In this activity (on page 14 of PDF), learners use a pan full of flour and some rocks to create a moonscape.

Nebula in a Jar
Source Institutions
In this activity, learners will build a model of a nebula using cotton balls and colored water. Astronomers photograph nebulas and add colors to provide information about the nebula's composition.

Your Body in Your Mind's Eye
Source Institutions
This activity is about how you form mental images of your body's position in space, independent of vision. Can you take a sip of water from a cup with your eyes closed?
Sound Dampeners
Source Institutions
In this activity, learners will experiment with water- and air-filled balloons as a way of dampening sound before it reaches their ears.

Geyser
Source Institutions
This Exploratorium activity can be used in many contexts because geysers are great opportunities for learning about heat and temperature changes as well as geological/space science phenomena.

Earthquake Science: Soil Liquefaction
Source Institutions
This activity demonstrates liquefaction, the process by which some soils lose their solidity during an earthquake.

Chemical Breath
Source Institutions
This is a chemistry lab activity about solutions (page 7 of the PDF). Learners see firsthand how chemicals in a solution can combine to form an entirely different substance.
