Search Results
Showing results 1 to 19 of 19

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).

Determining the Amount of Transpiration from a Schoolyard Tree
Source Institutions
In this activity, learners calculate the number of milliliters of water a nearby tree transpires per day.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!

Handwashing Laboratory Activities: Bowl Technique
Source Institutions
In this lab (Activity #2 on page), learners compare bacteria growth on two petri dishes containing nutrient agar. Learners touch the doors, faucets, etc.

Inflate-a-mole
Source Institutions
In this activity, learners conduct an experiment to find the volume of one mole of gas. Learners capture sublimated gas from dry ice in a ziploc bag and use water displacement to measure its volume.

Measure the Pressure: The "Wet" Barometer
Source Institutions
In this activity, learners use simple items to construct a device for indicating air pressure changes.
Without An Ark: The Effects of Storms and Floods
Source Institutions
April showers bring May flowers, but what do coastal storms bring?

Portable Potable Pressure
Source Institutions
In this activity, learners use plastic water bottles, wood, and water to build an inexpensive and portable tool to demonstrate one atmosphere of pressure at sea level.
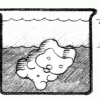
Gummy Growth
Source Institutions
In this activity related to Archimedes' Principle, learners use water displacement to compare the volume of an expanded gummy bear with a gummy bear in its original condition.

How Big is Small
Source Institutions
In this classic hands-on activity, learners estimate the length of a molecule by floating a fatty acid (oleic acid) on water.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.

Earth Atmosphere Composition
Source Institutions
In this activity, learners use rice grains to model the composition of the atmosphere of the Earth today and in 1880. Learners assemble the model while measuring percentages.

Handwashing Laboratory Activities: Fingerprint Technique
Source Institutions
In this lab (Activity #1 on page), learners compare bacteria growth on two petri dishes containing nutrient agar: one that has been touched by a finger washed only with water and one that has been tou

Linear Functions: Mystery Liquids
Source Institutions
In this math lesson, learners analyze the density of liquids in order to explore linear functions.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.
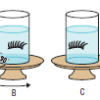
Visualizing How the Vestibular System Works
Source Institutions
In this activity (page 59 of the PDF), learners spin and observe false eyelashes in jars of water (prepared at least 1 day ahead of time) to investigate the effects of different types of motion on the
The Blue Crab's Chesapeake Journey
Source Institutions
In this data collection activity about crabs, learners use data from the Virginia Institute of Marine Science (VIMS) trawl survey to determine the areas of the Chesapeake Bay that are being used by bl

Wild Sourdough
Source Institutions
In this activity, learners explore chemistry and the microbial world by making their own sourdough starter and bread at home using only flour and water.

Mapping Sea Level Rise
Source Institutions
In this activity related to climate change, learners create and explore topographical maps as a means of studying sea level rise.
