Search Results
Showing results 101 to 118 of 118

Dissolving a Substance in Different Liquids
Source Institutions
In this activity, learners make colored sugar and add it to water, alcohol, and oil to discover some interesting differences in dissolving.
Milli's Super Sorting Challenge
Source Institutions
In this activity, learners separate materials based on their special properties to mimic the way recyclables are sorted at recycling centers.

Crash Landing!
Source Institutions
In this activity, groups cut out and sort cards showing items recovered from a crash landing on the Moon. The 12 items range from food and water to rope and matches to a self-inflating life raft.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.

Find the Best Way to Clean Oil off Bird Feathers
Source Institutions
In this experiment, learners examine the way oil affects bird feathers and test different cleanup methods to find out which works best.

Penny Battery
Source Institutions
In this activity, learners light an LED with five cents. Learners use two different metals and some sour, salty water to create a cheap battery.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.

Iridescent Art
Source Institutions
This is a quick activity (on page 2 of the PDF under Butterfly Wings Activity) that illustrates how nanoscale structures, so small they're practically invisible, can produce visible/colorful effects.

Rubber Blubber Gloves
Source Institutions
In this experiment, learners work in pairs to create two gloves -- one that contains a layer of shortening (blubber) inside, and one that doesn't.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.
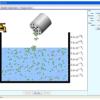
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.
