Search Results
Showing results 1 to 17 of 17

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Static Water
Source Institutions
In this activity, learners will use static elecricity to bend a stream of water without touching it. Learners will explore physics and cause and effect through this activity.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.
Cooling the Mummy's Tomb
Source Institutions
In this activity, learners conduct an experiment to help Pharaoh design a better insulated tomb.

Cool It!
Source Institutions
In this fun hands-on activity, learners use simple materials to investigate evaporation. How can the evaporation of water on a hot day be used to cool an object? Find out the experimental way!

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.
Making An Impact!
Source Institutions
In this activity (on page 14 of PDF), learners use a pan full of flour and some rocks to create a moonscape.

Wet Pennies
Source Institutions
Learners initially test to see how many drops of liquid (water, rubbing alcohol, and vegetable oil) can fit on a penny.

Critical Angle
Source Institutions
In this optics activity, learners examine how a transparent material such as glass or water can actually reflect light better than any mirror.

Iridescent Art
Source Institutions
This is a quick activity (on page 2 of the PDF under Butterfly Wings Activity) that illustrates how nanoscale structures, so small they're practically invisible, can produce visible/colorful effects.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.
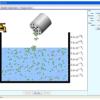
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.

Cook Up a Comet
Source Institutions
In this activity (on page 5 of PDF), learners use dry ice and household materials to make scientifically accurate models of comets.
