Search Results
Showing results 1 to 20 of 38

Breaking the Tension: Surface Tension 1
Source Institutions
Learners explore how the attractive forces between water molecules create surface tension and allow certain objects to float on the surface of water.

Moving On Up: Capillary Action 1
Source Institutions
Over the course of several days, learners explore the property of water that helps plants move water from roots to leaves or gives paper towels the capacity to soak up water.

A Little Drop of Water: Cohesion
Source Institutions
Learners explore water's property of cohesion through two investigations.

Stuck on You: Adhesion
Source Institutions
Learners explore water adhesion and learn about why water molecules are more strongly attracted to some substances than others.

What-a-cycle
Source Institutions
In this activity, learners act as water molecules and travel through parts of the water cycle to discover that it is more complex than just water moving from the ground to the atmosphere.
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.
What's So Special about Water: Absorption
Source Institutions
In this activity about water's cohesive and adhesive properties and why water molecules are attracted to each other, learners test if objects repel or absorb water.

Stick to It: Adhesion II
Source Institutions
Water sticks to all kinds of things in nature — flowers, leaves, spider webs - and doesn't stick to others, such as a duck's back.

Moving On Up: Capillary Action II
Source Institutions
Learners explore capillary action in plants (such as plants ability to move water from roots to leaves) in an investigation called Paper Blooms.

Differing Densities: Fresh and Salt Water
Source Institutions
In this activity, learners visualize the differences in water density and relate this to the potential consequences of increased glacial melting.

Above Water: Buoyancy & Displacement
Source Institutions
In an investigation called "Shape It!" learners craft tiny boats out of clay, set them afloat on water and then add weight loads to them, in order to explore: how objects stay afloat in water; what th

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Convection Current
Source Institutions
In this activity, learners make their own heat waves in an aquarium.
Aluminum Boats
Source Institutions
Test the buoyancy of an aluminum foil boat and an aluminum foil ball. Why does the same material in different shapes sink or float?
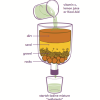
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

What is in the Water?
Source Institutions
In this activity, learners use open inquiry to learn about the process of science as well as gain experience regarding the Law of Conservation of Mass, dissolution, and density.

Below the Surface: Surface Tension II
Source Institutions
In this activity learners explore surface tension. Why are certain objects able to float on the surface of water and how do detergents break the surface tension of water?

The Liquid Rainbow
Source Institutions
Learners are challenged to discover the relative densities of colored liquids to create a rainbow pattern in a test tube.

Uplifting Force: Buoyancy & Density
Source Institutions
In this investigation, learners explore the force known as buoyancy by placing various objects into water and observing how they behave (for example, which sink more quickly, which float, how much wat

Oh Buoy!
Source Institutions
Learners work in pairs to design, construct, and test a device that exhibits positive, neutral, and negative buoyancy. They test a number of different objects in water to see if they sink or float.
