Search Results
Showing results 1 to 20 of 33

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Soap: Sometimes oil and water do mix!
Source Institutions
In this activity (on page 2 of PDF), learners mix oil and water. Then, they add soap and observe what changes! The activity demonstrates how oil and water don't mix, except when soap is added.

Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.
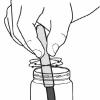
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Cool It!
Source Institutions
Learners make a refrigerator that works without electricity. The pot-in-pot refrigerator works by evaporation: a layer of sand is placed between two terra cotta pots and thoroughly soaked with water.
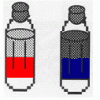
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

In the Toilet
Source Institutions
This activity explores the basic workings of a siphon, which is the core technology that makes toilets work.
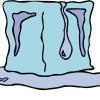
Ice Cube Painting
Source Institutions
In this activity, learners "draw" with frozen tempera paint. The ice cubes are prepared the day before by placing watered down tempera paint and popsicle sticks in ice cube trays.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.
Floating Golf Ball
Source Institutions
Visitors observe a graduated cylinder with a golf ball floating about halfway in liquid. The bottom half of the cylinder contains a concentrated solution of salt.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Phase Changes
Source Institutions
Learners observe a sealed test tube containing a small amount of solid stearic acid.

Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.
