Search Results
Showing results 1 to 20 of 95

Soapy Boat
Source Institutions
Learners discover that soap can be used to power a boat. Learners make a simple, flat boat model, put it in water, and then add a drop of detergent at the back of the boat.

Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.

Formation of a Precipitate
Source Institutions
Learners create hard water by mixing Epsom salt and water. Then they compare what happens when soap solution is mixed with hard water and regular water.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Release the Grease!
Source Institutions
In this simple activity (on page 7 of the PDF), learners use water and liquid dish detergent to see which one removes lipstick better from an index card.
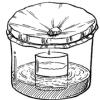
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.
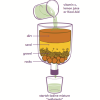
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Breathing Yeasties
Source Institutions
Does yeast breathe? Find out by watching how plastic bags filled with yeast, warm water and different amounts of sugar change over time.

Below the Surface: Surface Tension II
Source Institutions
In this activity learners explore surface tension. Why are certain objects able to float on the surface of water and how do detergents break the surface tension of water?

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Mixing and Unmixing in the Kitchen
Source Institutions
In this chemistry investigation, learners combine common cooking substances (flour, baking powder, sugar, salt, pepper, oil, water, food coloring) to explore mixtures.

Exploring Materials: Hydrogel
Source Institutions
In this activity, learners discover how a super-absorbing material can be used to move a straw.

Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Surface Tension
Source Institutions
In this activity exploring liquid dynamics, learners design and build a clay channel in a tray of water and then see what happens when food coloring and liquid soap are added to the mix.

Human Impact on Estuaries: A Terrible Spill in Grand Bay
Source Institutions
In this activity, learners make a model of a pollution spill that occurred at Bangs Lake in Mississippi and measure water quality parameters in their model.

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p
